
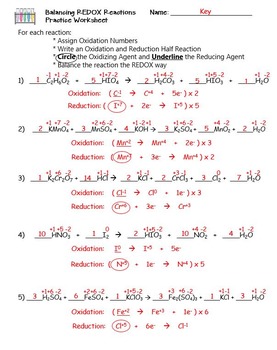
However there is an easier method which involves breaking a redox reaction into two half- reactions. Redox Balancing Worksheet Oxidation Reduction Packet Oxidation-reduction redox reactions are reactions in which oxidation numbers change.
Balance the following equations of redox reactions.
Balancing redox reactions worksheet. Balancing Redox Reactions Worksheet 1 Balance each redox reaction in. Mn 2 BiO3 -Æ MnO4 - Bi 3 MnO4 - S2O3 2- Æ S4O6 2- Mn 2. Worksheet 5 Balancing Redox Reactions in Acid and Basic Solution Balance each half reaction in basic solution.
Cr 2O 7 2 - Cr3 5. NO NO 3-6. SO 4 2- SO 2 7.
MnO 2 Mn 2O 3 Balance each redox reaction in acid solution using the half reaction method. H 2O 2 Cr 2O 7 2- O 2 Cr 3 9. TeO 3 2- N 2O 4 Te NO 3-10.
Balancing REDOX Reactions. Learn and Practice Reduction-Oxidation reactions or REDOX reactions occur when the chemical species involved in the reactions gain and lose electrons. Oxidation and reduction occur simultaneously in order to conserve charge.
We can see these changes if we assign oxidation numbers to the reactants and products. For rules about assigning oxidation. Redox Balancing Worksheet Oxidation Reduction Packet Oxidation-reduction redox reactions are reactions in which oxidation numbers change.
Oxidation numbers are either real charges or formal charges which help chemists keep track of electron transfer. Balancing redox reactions worksheet with solutions. Convert the following redox reactions to the ionic form.
Mn 2 bio3 æ mno4 bi 3 mno4 s2o3 2 æ s4o6 2 mn 2. Mno 2 mn 2o 3 balance each redox reaction in acid solution using the half reaction method. There are two common techniques for balancing redox equations.
Oxidation number change method ion-electron method also called the half-reaction method. Periodic table of the elements. Balance the following equations of redox reactions.
Assign oxidation numbers to all elements in the reaction. Balancing Redox Reactions Worksheets Last updated. Save as PDF Page ID 11048.
Contributed by Mark Draganjac. Professor Chemistry at Arkansas State University. Contributors and Attributions.
_____ Student ID_____ Work in groups on these problems. You should try to answer the questions without referring to your textbook. If you get stuck try asking another group for.
Balancing redox reactions worksheet 1 balance each redox reaction in. Teo 3 2 n 2o 4 te no 3 10. Convert the following redox reactions to the ionic form.
Balancing redox equations by the ion electron method. Mn 2 bio3 æ mno4 bi 3 mno4 s2o3 2 æ s4o6 2 mn 2. Balance redox equations using the ion electron method in an acidic solutions.
The steps for balancing redox reactions in basic solution. Complet and balance each reaction using the half-reaction method. Fe2 MnO 4-Fe3 Mn2 b.
Sn2 IO 3-Sn4 I-c. S2- NO 3-S NO d. NH3 NO2 N2 H2O e.
Mn2 BiO 3-Bi2 MnO 4-f. I 2 Na2S2O3 Na2S2O4 NaI. Determine what is oxidized and what is reduced in each reaction.
Identify the oxidizing agent and the. Balancing Redox Reactions Author. Todd Helmenstine Created Date.
8172008 15732 PM. To balance a redox equation by the ion-electron method carry out the following steps in this sequence. Separate the skeletal equation into two half reactions.
One half reaction will be a reduction and the other will be an oxidation. It is not necessary at this stage to identify which is which. Write balance equations for the following redox reactions.
NaBr Cl 2 NaCl Br 2 b. Fe 2 O 3 CO Fe CO 2 in acidic solution c. CO I 2 O 5 CO 2 I 2 in basic solution Hint.
Write balanced equations for the following reactions. CrOH 3 Br 2 CrO 4 2- Br-in basic solution. O 2 Sb H 2 O 2 SbO 2-in basic solution Hint.
HCOOH MnO 4-CO 2 Mn 2 in acidic solution. Some of the worksheets below are Redox Reactions Worksheets useful trick to help identify oxidation and reduction step by step guide to balance any Redox Equations explanation of Oxidation reduction oxidizing agent reducing agent and rules for assigning an oxidation number. Balancing Redox Reactions OxidationReduction Redox reactions can be balanced using the oxidation state changes as seen in the previous example.
However there is an easier method which involves breaking a redox reaction into two half- reactions. This is best shown by working an example. UNIT 6 REDOX REACTIONS 3 Lesson Title and Syllabus Reference 11 Electrolysis - Faradays Laws Applications CA4bi amount of substance mole of electrons.
CA10eii electrolysis factors influencing discharge of species Faradays Laws. Simple calculations based on the relation F Le 96500 C and mole ratios to determine mass volume of gases number of entities charges etc using.