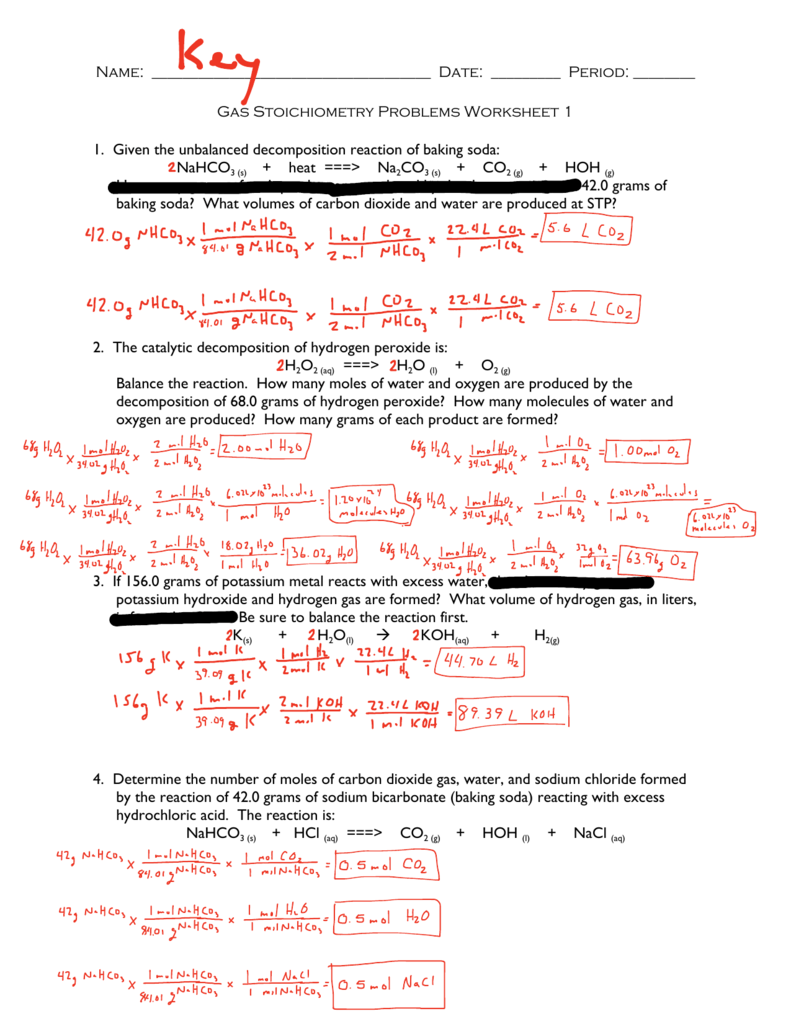
Question 1 Calcium carbonate decomposes at high temperatures to form carbon dioxide and calcium oxide. GAS STOICHIOMETRY WORKSHEET - MR Wu002639s GHHS Science Website GAS STOICHIOMETRY.

1 For the reaction 2 H2g O2g 2 H2Og how many liters of water can be made from 5 L of oxygen gas and an excess of hydrogen.
Gas stoichiometry practice sheet. Gas Stoichiometry Practice Sheet Answers 1For the reaction 2 H2g O2g 2 H2Og how many liters of water can be made from 5 L of oxygen gas and an excess of hydrogen. 10 L 5L O2 x 2L H2O1L O2. GAS STOICHIOMETRY WORKSHEET Please answer the following on separate paper using proper units and showing all work.
Please note that these problems require a balanced chemical equation. Carbon monoxide reacts with oxygen to produce carbon dioxide. I f 10 L of carbon monoxide reacts with oxygen at STP a.
How many liters of oxygen are required to react. How many liters of carbon. View GasStoichiometry from SCI 101 at Morgan County High School.
Gas Stoichiometry Practice Sheet 1 For the reaction 2 H2g O2g 2 H2Og how many liters of water can be made from 5. Gas Stoichiometry Practice Sheet For the reaction 2 H2g 02g 2 1-1206 how many liters of water can be made from 5 L of oxygen gas and an excess of hydrogen. AT STP 6 L C Hao I mol O I mol How many liters of water can be made from 55 grams of oxygen gas and 5 TP an excess of hydrogen at STP.
I mol OR L Il 3 a o - 7700 L I mol I HaO How many liters of water can be made from 55 grams of. Gas Stoichiometry Practice Sheet Answers. 1 For the reaction 2 H2g O2g 2 H2Og how many liters of water can be made from 5 L of oxygen gas and an excess of hydrogen.
2 How many liters of water can be made from 55 grams of oxygen gas and an excess of hydrogen at STP. 3 How many liters of water can be made from 55 grams of oxygen gas and an excess of hydrogen at a. Gas Stoichiometry Practice Sheet.
1 For the reaction 2 H2g O2g 2 H2Og how many liters of water can be made from 5 L of oxygen gas and an excess of hydrogen. 2 How many liters of water can be made from 55 grams of oxygen gas and an excess of hydrogen at STP. 3 How many liters of water can be made from 55 grams of oxygen gas and an excess of hydrogen at a pressure of 124 atm and.
Gas Stoichiometry Practice Sheet Answers. 1 For the reaction 2 H2g O2g 2 H2Og how many liters of water can be made from 5 L of oxygen gas and an excess of hydrogen. 2 How many liters of water can be made from 55 grams of oxygen gas and an excess of hydrogen at STP.
3 How many liters of water can be made from 55 grams of oxygen gas and an excess of hydrogen at a. Gas Stoichiometry Practice Sheet Answers Author. Jcaslick Last modified by.
1202010 55800 PM Company. Greater Essex School Board Other titles. Gas Stoichiometry Practice Sheet.
Gas Stoichiometry Practice Sheet Answers Read Gas Stoichiometry Practice Sheet Answers PDF on our digital library. You can read Gas Stoichiometry Practice Sheet Answers PDF direct on your mobile phones or PC. As per our directory this eBook is listed as GSPSAPDF-134 actually introduced on 9 Feb 2021 and then take about 2105 KB data size.
Gas Stoichiometry Practice Sheet. What do you get when you mix PV nRT with stoichiometry. The files above have been fully updated and upgraded.
Question 1 Calcium carbonate decomposes at high temperatures to form carbon dioxide and calcium oxide. CaCO 3s CO 2g CaO s How many grams of calcium carbonate will I need to form 345 liters of carbon dioxide at STP. Question 1 Calcium carbonate decomposes at high temperatures to form carbon dioxide and calcium oxide.
CaCO 3s CO. Gas Stoichiometry Practice Sheet For the reaction 2 H2g 02g 2 H20g how many liters of water can be made from 5 L of oxygen gas and an excess of hydrogen. ÖoL How many liters of water can e ma e rom grams of oxygen gas and an excess of hydrogen at STP.
How many liters of water can be made from 55 grams of oxygen gas and an excess of hydrogen at a pressure of 124 atm and a. About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy Safety How YouTube works Test new features Press Copyright Contact us Creators. STP refers to the term standard temperature and pressure and is equal to 273 K 0 C and a pressure of 1 atm.
1 mole of a gas is equivalent to 224 liters only at STP youll need to use PV nRT to figure out what it is for other conditions. Gas Stoichiometry Practice Sheet Answer Key This is likewise one of the factors by obtaining the soft documents of this gas stoichiometry practice sheet answer key by online. You might not require more era to spend to go to the book creation as well as search for them.
In some cases you likewise pull off not discover the proclamation gas. Gas Stoichiometry Practice Sheet Answers 1 For the reaction 2 H 2g O 2g Æ 2 H 2O g how many liters of water can be made from 5 L of oxygen gas and an excess of hydrogen. 10 L 2 How many liters of water can be made from 55 grams of oxygen gas and an excess of hydrogen at STP.
77 L 3 How many liters of water can be made from 55 grams of oxygen gas and an excess of hydrogen at a. I love the smell of stoichiometry in the morning. The most fun you can have with a calculator.
More Exciting Stoichiometry Problems. More fun for the whole chemist family. Balancing Equations and Simple Stoichiometry.
Just what it sounds like. This Gas Stoichiometry Practice Sheet Worksheet is suitable for 9th - 12th Grade. In this gas stoichiometry worksheet students determine the volume of gases in 4 problems given the temperature the pressure and the masses of the reactants.
They find the limiting reactant in one problem at STP. Gas Stoichiometry Practice Sheet Work. June 22nd 2013 124830 PM.
Gas Stoichiometry Practice Gas Stoichiometry Practice For all of these problems assume that the reactions are being performed at a pressure of 10 atm and a temperature of 298 K. PRA036pdf - Read File Online - Report Abuse. GAS STOICHIOMETRY WORKSHEET - MR Wu002639s GHHS Science Website GAS STOICHIOMETRY.